Noon Energy, an American cleantech company founded in 2018, develops low-cost, high-energy density rechargeable flow batteries for renewable energy storage. The battery stores excess solar and wind electricity through splitting the carbon dioxide (CO₂) storage medium into solid carbon and oxygen gas.
Challenges: renewable energy storage
Solar cells and wind turbines are increasingly being used to generate clean electricity. Ideally, all of the electricity they intermittently produce would be used. The excess electricity should be stored. The ability to store their electrical energy would significantly increase the efficiency and reliability of these intermittent renewable energy sources as base load supply. Batteries are strong candidate solutions owing to their small spatial footprint, mechanical simplicity, and flexibility in siting.
Lithium-ion batteries are a mature technology. They store electrical energy in expensive electrodes made of lithium, cobalt, etc. The high cost of lithium-ion batteries prevents their widespread use to store energy on the grid. Additionally, grid energy storage batteries must be safe. It has been reported that several dozens of lithium-ion energy storage systems have resulted in explosions or fires. Therefore, there is a need for low-cost and safe battery technology for grid-scale energy storage.
Flow batteries store electrical energy in tanks of liquid electrolyte with dissolved ionic species, such as vanadium ions, that are pumped through electrodes. During charging, the pumps push spent electrolyte through the electrodes, where the electrolyte is recharged and returned to the holding tank. Thus, the storage capacity and energy density depend on the size of the storage tank. Increasing the capacity of batteries to store more energy simply requires bigger tanks of electrolytes.
However, the current flow batteries have several disadvantages:
- Large tanks are needed to store the electrolyte, making their energy density low;
- The flow controlling system comprises a number of compressors, expanders, condensers, and pumps to ensure the flow of electrolytes to and from the battery cell, resulting in a very complex system;
- The flow controlling system is expensive and typically dominates the system cost of a flow battery; and
- The flow controlling system also consumes energy, reducing the efficiency of the flow battery and increasing the energy storage cost.
Noon Energy Technology
Noon Energy develops rechargeable carbon-oxygen flow battery systems with improved energy density and energy efficiency. The flow battery system uses carbon dioxide (CO₂) instead of a liquid electrolyte as the energy storage medium. The flow battery system stores energy by splitting CO₂ into solid carbon (C) and oxygen (O₂), resulting in a high energy density of around 300 Wh/kg (650 Wh/L).
Moreover, the flow battery is a passive flow battery, which eliminates the need for fluid controlling components, thereby reducing the complexity and cost of the system. The flow battery system is 80% less expensive than state-of-the-art lithium ion batteries with storage capacities greater than 8 hours.
Noon Energy flow battery
The diagram below depicts the structure of Noon Energy’s rechargeable carbon-oxygen flow battery system.
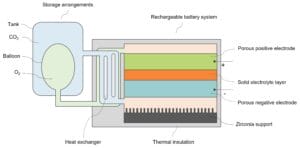
The flow battery system comprises thermal insulation, a porous negative electrode, a porous positive electrode, a solid electrolyte layer separating the two electrodes, a heat exchanger, and storage arrangements for carbon dioxide and oxygen.
- Thermal insulation
The flow battery is operated at high pressures and temperatures ranging from 50 to 200 bar and 600 to 800 ºC, respectively, to permit efficient chemical reactions in electrodes and high ionic migration within the electrolyte layer. The thermal insulation maintains the battery temperature and improves its energy efficiency.
- Porous negative electrode
During charging, the porous negative electrode reduces CO₂. It is permeable to CO₂ gas diffusion. The electrode contains a catalyst material, such as doped ceria (CeO₂), which catalyzes exclusively the reduction of CO₂ to carbon monoxide (CO) and oxygen ions (O²⁻).
The decomposition of CO into solid carbon nanofibers or multi-walled carbon nanotubes occurs on the second catalytic material, such as nickel nanoparticles, loaded on a nearby zirconia support. The zirconia support improves the thermal stability of nickel nanoparticles. The surface of catalysts is coated with molten lithium carbonate (Li₂CO₃) to improve robustness for reversible carbon deposition. During reversible deposition cycles, the mobility of the molten phase also helps maintain the wetting of the catalyst and carbon. The surface coating of molten carbonate also accelerates the reactions.
- Solid electrolyte layer
The electrolyte allows fast oxygen ion migration at high temperature. The thickness of the electrolyte layer is between 10 and 100 microns. The electrolyte is made of solid oxide oxygen ion conductors, molten metal carbonates, molten hydroxides, or solid oxide proton conductors.
- Porous positive electrode
During charging, the porous positive electrode oxidizes oxygen ions to O₂. It is permeable to O₂ diffusion. The positive electrode comprises a catalyst for promoting O₂ formation.
- Heat exchanger
During charge mode, the heat exchanger heats the cold CO₂ flowing from CO₂ tank towards the negative electrode with hot O₂ flowing away from the positive electrode .
- Storage arrangements
The storage arrangement comprises a tank for storing CO₂ reactant and an inflatable balloon inside the tank for storing O₂ products. The CO₂ tank and O₂ balloon are connected via valves to the battery’s negative and positive electrodes, respectively. Thus, the battery and storage arrangements form a closed system that enables a gaseous flow from the CO₂ storage and to the O₂ storage during the charging process, while maintaining constant total pressure and volume of the gaseous reactant (CO₂) and product (O₂).
The pressures of CO₂ and O₂ are equalized by allowing the volume of the CO₂ tank to vary with an inflatable O₂ balloon inside the CO₂ tank, thereby eliminating the need for a pressure regulating system. If the O₂ was externally from the air, a compressor would be needed to compress the air, and a pressure regulator to control a large pressure gradient from CO₂ side of the battery to the air side would be needed. Thus, the combined storage arrangement of the CO₂ tank with the inflatable O₂ balloon within the tank facilitates a simple, volume efficient, and energy efficient flow battery system.
The above-described carbon-oxygen flow battery stores a mixture of CO₂ and CO. CO is toxic. To improve the safety of the flow battery system, Noon Energy designs an alternative carbon-oxygen flow battery that stores pure CO₂, as depicted in the diagram below.

This type of flow battery has a CO₂ separation membrane between the CO₂ tank and the battery’s reaction zone. The membrane selectively permeates CO₂ so that the CO₂/CO gas mixture only remains within the insulated hot zone where the electrochemical and thermochemical reactions are occurring.
All components of the battery system are arranged inside a single tubular tank, which simplifies and minimizes sealing between the CO₂ and O₂ sides of the cells. High quality sealing provides a low battery self-discharge rate.
How Noon Energy flow battery works
The diagram below depicts the charging process of the carbon-oxygen flow battery.

When the flow battery is fully discharged, the CO₂ tank is filled with 93% CO₂ and 7% CO at 200 bar . The gasses are stored at a temperature of 34 ºC. The battery maintains a high temperature between 600 and 800 ºC.
When a voltage of 1.08 V is applied during charging, CO₂ from the tank enters the negative electrode. CO₂ is electrochemically reduced in the porous negative electrode, forming CO and oxygen ions (O²⁻). CO diffuses to the nearby zirconia support loaded with nickel nanoparticle catalysts, where it is thermochemically reduced to solid carbon nanofibers or multi-walled carbon nanotubes and CO₂. CO₂ diffuses back to the negative electrode for electrochemical reaction. The net reaction in the negative electrode is CO₂(g) + 4e⁻ → C(s) + 2O²⁻. Oxygen ions diffuse through the electrolyte layer to the positive electrode and are oxidized to O₂.
The CO reduction at zirconia support is exothermic. Heat released from this reaction can be absorbed by the CO₂ electrolysis reaction in the negative electrode and oxygen ion oxidation in the positive electrode. Both reactions are endothermic. Reaction heat can easily transfer from the zirconia support to the positive electrode because the electrolyte layer is very thin (no more than 100 microns).
During charging, the consumption of CO₂ forms a concentration gradient between the negative electrode and the CO₂ tank, causing CO₂ to flow into the negative electrode. The production of O₂ generates a concentration gradient between the positive electrode and the balloon, causing O₂ to flow into the balloon. Thereby during charging, the CO₂ pressure decreases and the O₂ pressure increases. The combined storage arrangement allows the volume of the CO₂ tank to vary with an inflatable balloon of O₂ inside the CO₂ tank, resulting in an automatic flow of reactants and products within the system without the need of pressure regulating means.
During discharging, the flows to and from the negative and positive electrodes will be reversed.
Noon Energy Patent
- US20200358123A1 Passive flow battery
- WO2019141773A1 Passive flow battery
- EP3740601A1 Passive flow battery
- US9780424B2 Rechargeable carbon-oxygen battery
- WO2014044285A1 A rechargeable carbon-oxygen battery
- EP2898564B1 A rechargeable carbon-oxygen battery
- DK2898564T3 Genopladeligt carbon/oxygen-batteri
- KR102162095B1 충전가능 카본-산소 배터리
- CN104798247A 一种可充电的碳-氧电池
- JP2015534220A 充電式炭素−酸素電池
Noon Energy Battery Applications
Noon Energy’s battery technology is primarily aimed at transforming the renewable energy sector. The battery’s ability to store energy by splitting CO₂ into solid carbon and oxygen makes it suitable for grid-scale storage of renewable energy. This can help in making solar and wind electricity available on-demand, thus overcoming the challenge of intermittency.
Noon Energy Products
Noon Energy’s carbon-oxygen flow battery could provide long-term stationary storage of excess solar and wind power at much lower cost than lithium-ion batteries. It will make renewable energy always available, 24/7 year-round, at a lower cost than fossil fuel generation and with zero emissions.
Noon Energy Funding
Noon Energy has raised a total of $31.2M in funding over 4 rounds:
- a Grant round
- a Non-equity Assistance round
- a Seed round
- a Venture – Series Unknown round
Their latest funding was raised on Dec 14, 2022 from a Venture – Series Unknown round.


Noon Energy Investors
Noon Energy is funded by 10 investors:
- Clean Energy Ventures
- Saudi Aramco Energy Ventures
- Aramco Ventures
- Prime Impact Fund
- Collaborative Fund
- PRIME Coalition
- At One Ventures
- Xplorer Capital
- National Science Foundation
- Cleantech Open
Aramco Ventures and Clean Energy Ventures are the most recent investors.

Noon Energy Founder
Christopher Graves is Founder.
Noon Energy CEO
Christopher Graves is CEO.
Noon Energy Board Member and Advisor
Jonathan Bonanno is an advisor.
Tibor Toth is a board member.