Hystar, a Norway cleantech startup founded in 2020, develops PEM electrolyzers for large-scale green hydrogen production. The company’s PEM electrolyzer uses a thin proton exchange membrane below 30 microns and consumes humidified air and liquid water to produce hydrogen. This design results in 20% less energy consumption and a low-cost green hydrogen.
Challenges: hydrogen fuel
Electrolysis of water (H₂O) can produce hydrogen gas (H₂) using an electrolyzer, such as proton exchange membrane (PEM) electrolyzer. The PEM is preferably made of perfluorosulfonic acid (PSFA) polymers, such as Nafion® or Aquvion®. The diagram below schematically depicts the structure and operation of a conventional PEM electrolyzer.
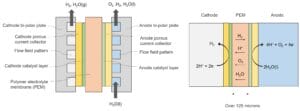
During operation, H₂O must be supplied continuously to the anode, where H₂O is oxidized to oxygen gas (O₂), protons (H⁺), and electrons (e⁻). An electric field across the PEM causes protons to migrate from the anode to the cathode. At the cathode, protons combine with electrons transferred through an external circuit to produce H₂. Produced H₂ and O₂ can diffuse through the membrane due to their partial pressure gradient across the membrane. A negligible amount of H₂ in O₂ in the anode can form explosive gas mixtures. In this regard, conventional PEM electrolyzers use PEM that is thicker than over 125 microns (μm) to effectively reduce H₂ and O₂ diffusion through the membrane.
However, this thick PEM limits the rate of gas generation, which is governed by Faraday’s law. An increase in the current passing through the electrolyzer increases the gas production and H₂O consumption proportionally. However, the use of such a thick membrane results in a significant ohmic resistance and, consequently, a decrease in the electrolyzer’s efficiency, particularly at current densities above 1 A cm⁻².
Currently, water electrolyzers are operated with a stack efficiency around 65-70% (higher heating value HHV), resulting in a power demand of about 55 kWh per kilogram of H₂. About 50 kWh are consumed by the electrolysis process, while the remaining 5 kWh are utilized by circulation and feed water pump, heat exchanger, ion exchanger, gas/water separators, valves and sensors, etc.
Therefore, an increase in the efficiency of the electrolyzer stack will rescue both the total amount of primary electrical energy consumption and the total cost of H₂.
Hystar Technology
Hystar has developed more efficient PEM electrolyzers simply by reducing the thickness of PEM to below 35 microns (μm) and supplying humidified air to the anode and H₂O to the cathode. The use of a thin PEM reduces the ohmic resistance of the electrolysis cell, thereby decreasing the energy consumption of the process by 15-20%. Supplying humidified air to the anode and H₂O to the cathode eliminates the risk of explosive gas mixtures and improves the stability of the electrolyzer.
Hystar electrolyzer
The diagram below schematically depicts the structure and operation of Hystar’s PEM electrolyzer.
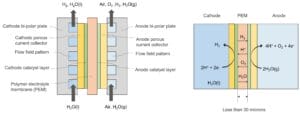
Except for a thin PEM, Hystar’s PEM electrolyzer has the same components as a conventional PEM electrolyzer. The Hystar’s PEM electrolyzer comprises the following components:
- Anode
The anode consists of a bi-polar plate, a metallic porous transport layer, and a catalyst layer.
The anode bi-polar plate is made of a highly corrosion-resistant and electrically conductive metallic material. It has a flow field pattern with inlet and outlet flow distribution manifolds for optimal O₂ and humidified air distribution along the active area of the anode. The relative humidity and temperature of humidified air supplied to the anode are above 75% and between 50 and 90 ºC, respectively.
The anode metallic porous transport layer is made of a highly corrosion-resistant and electrically conductive porous material that permits the diffusion of humidified air into the anode catalyst layer.
The anode catalysts layer comprises a catalyst that is highly efficient for the O₂ evolution reaction.
- Cathode
The cathode comprises a bi-polar plate, a metallic porous transport layer, and a catalyst layer.
The cathode bi-polar plate is also made of a highly corrosion-resistant and electrically conductive metallic material. It has a flow field pattern with inlet and outlet flow distribution manifolds for optimal liquid H₂O and H₂ distribution along the active area of the cathode.
The cathode metallic porous transport layer is made of a highly corrosion-resistant and electrically conductive porous material that enables the transport of H₂O and H₂ gas into and out of the cathode catalyst layer.
The cathode catalysts layer comprises a catalyst that is highly efficient for the H₂ evolution reaction.
- PEM
PEM has a thickness between 10 and 35 microns. Both sides of PEM are coated with the anode catalyst layer and the cathode catalyst layer, respectively. It permits the migration of protons, H₂, and H₂O vapor across the membrane.
How does the Hystar’s PEM electrolyzer operate?
During operation, ion exchanged H₂O is introduced to the cathode. Humidified air is supplied to the anode. A portion of H₂O on the cathode is absorbed by the PEM and moves to the anode. On the anode, H₂O reacts and is converted to O₂, protons, and electrons:
2H₂O → 4e⁻ + 4H⁺ + O₂
An electric field across the membrane induces protons to migrate from the anode side to the cathode side. Meanwhile, protons drag a significant portion of liquid H₂O from the anode side to the cathode side. At the cathode, protons combine with electrons transferred through an external circuit to produce H₂:
2e⁻ + 2H⁺ → H₂
Produced H₂ and O₂ can diffuse through the membrane due to their partial pressure gradient across the membrane. Humidified air along with O₂ and small amounts of H₂ exit the anode of the electrolyzer. H₂ along with the excess H₂O and traces of O₂ exit the cathode of the electrolyzer.
Why is the Hystar’s PEM electrolyzer more efficient?
Applying a large cell current can increase the gas generation and H₂O consumption rates. Therefore, sufficient H₂O must be supplied to the anode.
The thin PEM of Hystar electrolyzer not only allows a larger limiting cell current density, but also increases the H₂O transport from the cathode to the anode, resulting in higher H₂ and O₂ generation rates. In addition, the cathode pressure is controlled to be between 0.5 bar to 35 bar higher than the anode pressure. This pressure differential pushes and improves H₂O transport from the cathode to the anode, resulting in an increased gas production rate.
How does Hystar solve the safety problem?
The thin PEM also increases the flux of H₂ from the cathode to the anode and O₂ from anode to cathode, which increases the risk of explosive or flammable gas mixture formation. Hystar avoids this risk by supplying humidified air to the anode. Humidified air effectively dilutes the H₂ in the anode to levels well below the lower explosion limit of hydrogen-air mixtures, which is about 4 mol-%.
How about the stability of Hystar’s PEM electrolyzer?
PEM can be attacked and degraded by hydrogen peroxide (H₂O₂) and free radicals, which are byproducts of the reduction of O₂ at the cathode. The formation rate and concentration of free radicals in the membrane are directly proportional to the O₂ diffusion flux from the anode to the cathode. The diffusion rate is generally directly proportional to the partial pressure of O₂ in the anode, whereas the convection rate is proportional to the H₂O flux through the membrane.
In Hystar’s PEM electrolyzer, as the anode is fed with humidified air, the combination of nitrogen (N₂) and H₂O vapor in the air provides a much lower partial pressure of O₂ than that in a conventional PEM electrolysis cell. In addition, the net H₂O flux in Hystar’s PEM electrolyzer is from the cathode to the anode, whereas it is from the anode to the cathode in a conventional PEM electrolyzer. Thus, compared to conventional PEM electrolyzers, Hystar’s PEM electrolysers with humidified air feed on the anode and water feed on the cathode have a significantly lower rate of free radicals formation and membrane degradation.
Hystar electrolyzer system
The diagram below schematically depicts Hystar’s PEM electrolyzer system.
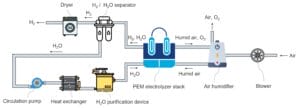
Humidified air with controlled humidity from an air humidifier is supplied to the anode of the electrolyzer stack so that it is evenly distributed over the active surface of the anode and dilutes H₂ permeating from the cathode to a level below 1 volume% in the anode. The cathode is supplied with ion exchanged H₂O from a H₂O purification device so that sufficient H₂O diffuses to the anode for the O₂ evolution reaction.
Produced H₂ along with H₂O exit the electrolyzer stack. H₂ and H₂O are separated in a H₂/H₂O separator. H₂ passes through a dryer. The separated H₂O is recycled to the H₂O purification device via a circulation pump and a heat exchanger and then into the electrolyzer stack.
Hystar Patent
- US20220364246A1 Method for producing hydrogen in a pem water electrolyser system, pem water electrolyser cell, stack and system
- US11408081B2 Method for producing hydrogen in a PEM water electrolyser system, PEM water electrolyser cell, stack and system
- EP3649276B1 A method for producing hydrogen in a pem water electrolyser system, pem water electrolyser cell, stack and system
- CA3068413A1 A method for producing hydrogen in a pem water electrolyser system, pem water electrolyser cell, stack and system
- HUE056264T2 A method for producing hydrogen in a pem water electrolyser system, pem water electrolyser cell, stack and system
- CN111051573B 在pem水电解器系统中生产氢的方法,pem水电解器单元、堆叠体和系统
- SI3649276T1 Postopek za pridobivanje vodika v PEM-vodnem elektroliznem sistemu, PEM-vodna elektrolizna celica, sklad in sistem
- DK3649276T3 A method for producing hydrogen in a pem water electrolyser system, pem water electrolyser cell, stack and system
- HRP20211658T1 Postupak za proizvodnju vodika u sustavu elektrolizera vode s pem, ćelija elektrolizera vode s pem, stog i sustav
- ES2898858T3 Un método para producir hidrógeno en un sistema electrolizador de agua PEM, celda de electrolizador de agua PEM, pila y sistema
- RS62621B1 Postupak za proizvodnju vodonika u pem sistemu za elektrolizu vode, pem ćelija elektrolizera vode, stroj i sistem
- AU2018297890A1 A method for producing hydrogen in a pem water electrolyser system, pem water electrolyser cell, stack and system
- PL3649276T3 Metoda wytwarzania wodoru w układzie elektrolizera wody pem, ogniwa elektrolizera wody pem, stosu i układu
- JP2020525653A5 PEM水電解槽システム、PEM水電解槽セル(又は電池;cell)、スタック及びシステムにて水素を生成するための方法
- PT3649276T Método para produzir hidrogénio num sistema eletrolisador de água de membrana eletrolítica polimérica (pem), sistema, pilha, célula eletrolítica de água de pem
- NO343985B1 Polymer electrolyte membrane (PEM) water electrolyser cell, stack and system and a method for producing hydrogen in said PEM water electrolyser system
Hystar Technology Applications
Green hydrogen
Hystar has a three-year supply agreement with Johnson Matthey to ramp up green hydrogen production. Furthermore, the company has collaborated with Semcon to develop new technology for hydrogen gas production, with the goal of supporting future energy needs.
Hystar Products
Hystar offers two product lines of PEM electrolyzer systems: Vega™ and Mira™. Vega™ has the best system efficiency and a 10% decrease in energy consumption, while Mira™ has the highest production rates and a 150% increase in production rates. The installation of both systems starts at 1 MW. Both systems can produce high purity H₂ (99.97%) at an outlet pressure of 4.0 bar and a temperature of 30 ºC.
Hystar Funding
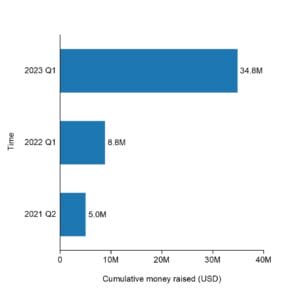
Hystar has raised a total of $34.8M in funding over 3 rounds:
- a Venture-Series Unknown round
- a Grant round
- a Series B round
Their latest funding was raised on Jan 11, 2023 from a Series B round.

Hystar Investors
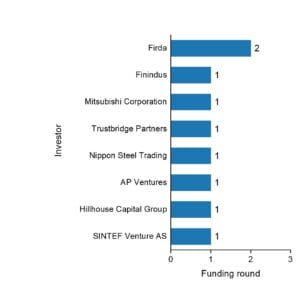
Hystar is funded by 8 investors:
- Firda
- Finindus
- Mitsubishi Corporation
- Trustbridge Partners
- Nippon Steel Trading
- AP Ventures
- Hillhouse Capital Group
- SINTEF Venture AS
Mitsubishi Corporation and Trustbridge Partners are the most recent investors.
Hystar Founder
Alejandro Oyarce Barnett and Magnus Thomassen are Co-Founders.
Hystar CEO
Fredrik Mowill is CEO.
Hystar Board Member and Advisor
Magnus Nordseth and Charlie Clark are board members.