Boston Metal, an American cleantech company founded in 2012, decarbonizes steel production using clean electricity. Their technology, called Molten Oxide Electrolysis (MOE), has received significant investment from companies like Microsoft and ArcelorMittal. Boston Metal’s MOE process is coal-free and emissions-free, and it has the potential to eliminate around 8% of global carbon dioxide emissions. The company funding rounds and investors are shown below.
Challenge: steel industry carbon emissions
The steel industry is highly emission-intensive, and it generates around 8% of global carbon dioxide CO₂ emissions. The total CO₂ emissions from the iron and steel sector have risen over the past decade, largely due to increases in steel demand and the required energy for production. Every ton of steel produced in 2018 emitted on average 1.85 tons of CO₂, equating to about 8% of global carbon dioxide emissions. Steelmaking releases more than three billion metric tons of CO₂ each year, making it the industrial material with the biggest climate impact.
The production of steel involves a complex process that includes mining, smelting, and refining of iron ore to produce steel. The process generates significant amounts of CO₂ emissions, both directly and indirectly. Direct emissions occur from the burning of fossil fuels, primarily coal and natural gas, to power the steel production process. Indirect emissions are generated from the production of electricity used in the steel production process, which is also generated from the burning of fossil fuels.
Efforts have been made to reduce carbon emissions in the steel industry through the adoption of cleaner technologies and the use of alternative energy sources. CO₂ emissions from the world’s steel sector will fall by nearly one-third by 2050 compared with last year as more companies adopt low-carbon technologies.
One such technology is carbon capture and storage (CCS), which involves capturing carbon dioxide emissions from the steel production process and storing them underground. This technology is still in the early stages of development and implementation, but it has the potential to significantly reduce carbon emissions in the industry.
Another approach to reducing carbon emissions in the steel industry is the use of renewable energy sources such as solar and wind power. Some steel manufacturers are exploring the use of renewable energy sources to power their operations, which could help reduce their reliance on fossil fuels and lower their carbon footprint.
In addition to these technological solutions, there is also a growing trend towards circular economy practices in the steel industry. This involves the recycling of scrap metal to produce new steel, reducing the need for new raw materials and energy-intensive processes. Recycling steel can also significantly reduce carbon emissions as the production of recycled steel requires much less energy compared to the production of new steel.
Boston Metal Technology
Boston Metal’s technology, called Molten Oxide Electrolysis (MOE), uses renewable electricity to convert crude iron ore directly into high-purity molten iron in a one-step process. This process avoids CO₂ emissions from primary steel production and does not generate waste, offering a simple, scalable, and truly decarbonized solution. MOE is an electrolytic cell that uses electricity to process raw iron ore instead of carbon. In MOE, the iron ore is melted, and an electric current is passed through it to separate oxygen from the iron. The result is high-purity molten iron that can be used for steel production without generating any CO₂ emissions.
Boston Metal MOE
The diagram below shows the structure of the molten oxide electrolysis (MOE) cell.

The electrolysis cell mainly comprises a gas seal, vessel lid, refractory vessel, an anode, and a current collector with conductive extension at the base of the refractory vessel.
- Gas seal
The gas seal is coupled with the vessel lid. The gas seal limits gas released from the refractory vessel and allows the movement of anode.
- Vessel lid
The removable lid is coupled with the refractory vessel to form a seal with the vessel.
The vessel lid has an exhaust aperture for distributing gas from the refractory vessel.
The lid also has feed apertures to introduce fine particles of ore, metal oxide electrolyte, and other additives into the refractory vessel. Boston Metal’s system facilitates continuous materials processing, whereas many conventional high-temperature reactors only operate in batch processing.
The lid also has injection apertures as well as sensing apertures (not shown).
The access ports on the lid are threaded so that they can be sealed with a cap during operation to restrict or prevent gas release.
- Refractory vessel
The refractory vessel has three layers: the exterior, the interior, and the intermediate layer.
The exterior layer is made of insulating material to reduce heat loss. A high temperature oxide electrolyte contacts the interior layer of the refractory vessel. The interior layer is therefore composed of a substance that is chemically compatible with an electrolyte. The material is resistant to extreme temperatures, pressure, and chemicals. The intermediate layer gives the refractory vessel stability in terms of structure, temperature, or other characteristics.
- Current collector
The current collector consists of a conductive bar coupled with the base of the refractory vessel. Current collector comprises an array of conductive extensions positioned centrally within the plurality of apertures in the base of the refractory vessel.
The diagram below details the structure of the current collector.
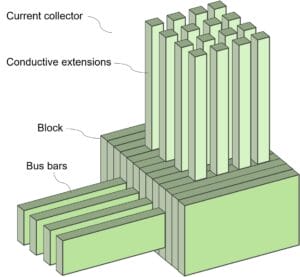
The current collector comprises a block which has bus bars to provide electrical communication from the MOE cell. Many conductive extensions protrude from the block.
By adjusting the number and position of the conductive elements, the system can be adjusted in a variety of ways to achieve stability or equilibrium within the vessel.
The conductive extensions are made of a metal with a lower melting point than the material being refined in the vessel. Due to the relatively stable temperature above the melting point of the material within the vessel, heat is transmitted through the refractory base and the conductive extensions. This results in the melting of at least a portion of the conductive extensions. This liquid material fills in any interstitial space between the conductive extensions and the aperture before being re-solidified within apertures as the metal moves further from the thermal center. This prevents molten material from escaping through the aperture to the collector, which could cause a system failure.
How Boston Metal MOE works
The diagram below shows the working mechanism of the molten oxide electrolysis cell.
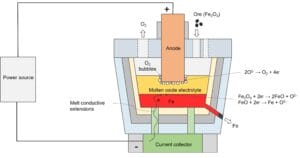
An initial amount of processable material is added to the vessel. The material consists of an ore, electrolyte, and additives. The ore mainly contains Fe₂O₃. The molten oxide electrolyte comprises at least three of cerium oxide, lanthanum oxide, strontium oxide, barium oxide, beryllium oxide, magnesium oxide, and calcium oxide to form a relatively stable supporting electrolyte at a high temperature of 1,600 ºC. The proportions of electrolyte mixtures are selected to match the required physical and chemical properties for extraction of iron metal via molten oxide electrolysis (MOE).
In molten oxide electrolysis processing, current passes through the anode into the molten oxide electrolyte near the anode, then into the reduced liquid metal at the bottom of the vessel, and finally to the current collector block via liquid conductive extensions.
Transporting between approximately 1,000 and 5,000 amperes of current through the vessel will generate Joule heating. The generated heat causes the materials within the vessel to melt. Oxygen ions (O²⁻) flow towards anode and are oxidized to produce oxygen gas (O₂) bubbles. Oxygen gas is released. Metal ions are reduced as they flow towards the current collector. The metal that initially forms close to the refractory base is molten and negatively charged; it will act as the cathode of the MOE cell. This negatively charged, molten metal is refined by the process and taped out from the vessel through ports.
During tapping operations, the level of material within the refractory vessel is lowered, and the anode is lowered as well to maintain a reaction during tapping, thus, realizing a continuous production.
Boston Metal Patent
- WO2018031984A1 Leak free current collector assemblage for metallurgical vessel and methods of manufacture
- US20200263313A1 Systems and methods for molten oxide electrolysis
- US20200248326A1 Electrolytic production of reactive metals
Boston Metal Technology Applications
- Decarbonizing steel production
Boston Metal’s technology provides a direct, scalable solution to make green steel. It uses an electro-chemical process where an inert anode is immersed in an electrolyte containing iron ore and then electrified. This results in a clean, high purity liquid metal that can be sent directly to ladle metallurgy. This process eliminates the need for coal in steel production, thereby reducing carbon emissions. The electro-chemical cell is modular and about the size of a school bus, allowing for scalability from thousands to millions of tons of output by adding new cells.
- Recovering high-value metals from mining waste
Boston Metal’s technology is also used to extract valuable metals from complex, low-concentration materials that are currently considered waste. This enables mining companies to reduce the financial and environmental liabilities of slag by leveraging a natural by-product of metal production to create new revenue streams.
Boston Metal Product
Boston Metal’s modular MOE cells are about the size of a school bus and can be scaled — from thousands to millions of tons of output — simply by adding new cells.
Compared to traditional production methods, Boston Metal’s technology offers a more efficient, lower cost, and environmentally sustainable solution across the steel value chain. It’s competitive without a carbon tax.
Boston Metal molten oxide electrolysis technology is on track to reach commercialization by 2026 to meet growing global demand for green steel, and our high-value metals business will produce revenue in 2023.
Boston Metal Funding
Boston Metal has raised a total of $332.7M in funding over 9 rounds:
Their latest funding was raised on Sep 6, 2023 from a Series C round.


Boston Metal Investors
Boston Metal is funded by 21 investors:
- Prelude Ventures
- Breakthrough Energy Ventures
- Climate Investment
- The Engine
- National Science Foundation
- BHP Ventures
- Microsoft Climate Innovation Fund
- OGCI Climate Investments
- Energy Impact Partners
- BMW i Ventures
- Piva Capital
- Devonshire Investors
- ArcelorMittal
- Vale
- FMR Computers and Software
- SiteGround Capital
- International Finance Corporation
- Baillie Gifford
- BloombergNEF
- M&G Investments
- Aramco Ventures
Baillie Gifford and Microsoft Climate Innovation Fund are the most recent investors.

Boston Metal Founder
Donald Sadoway is Founder.
Boston Metal CEO
Tadeu Carneiro is CEO.
Boston Metal Board Member and Advisor
Mark Cupta, Rick Cutright, Christopher Poirier are Board Member.